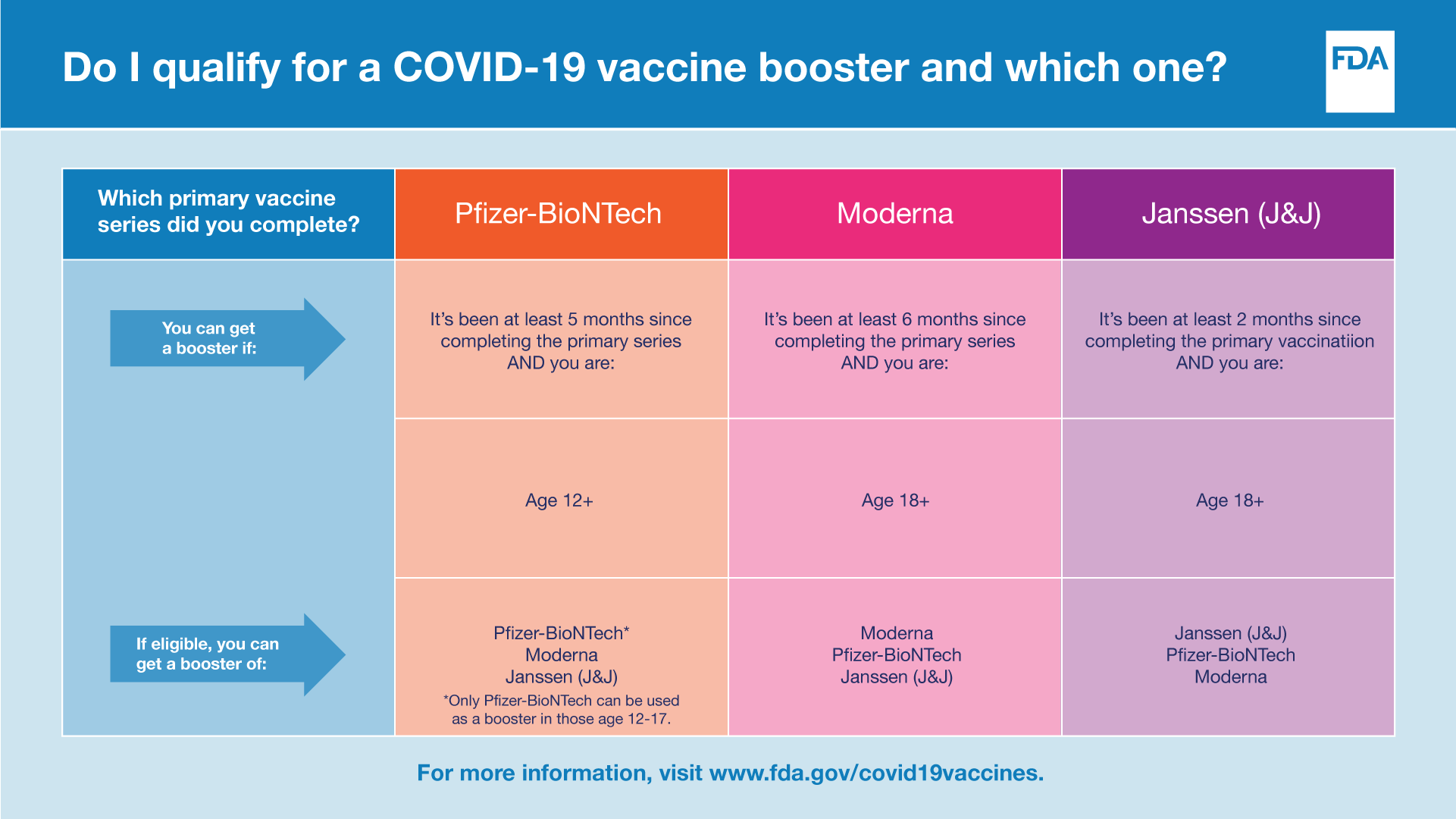
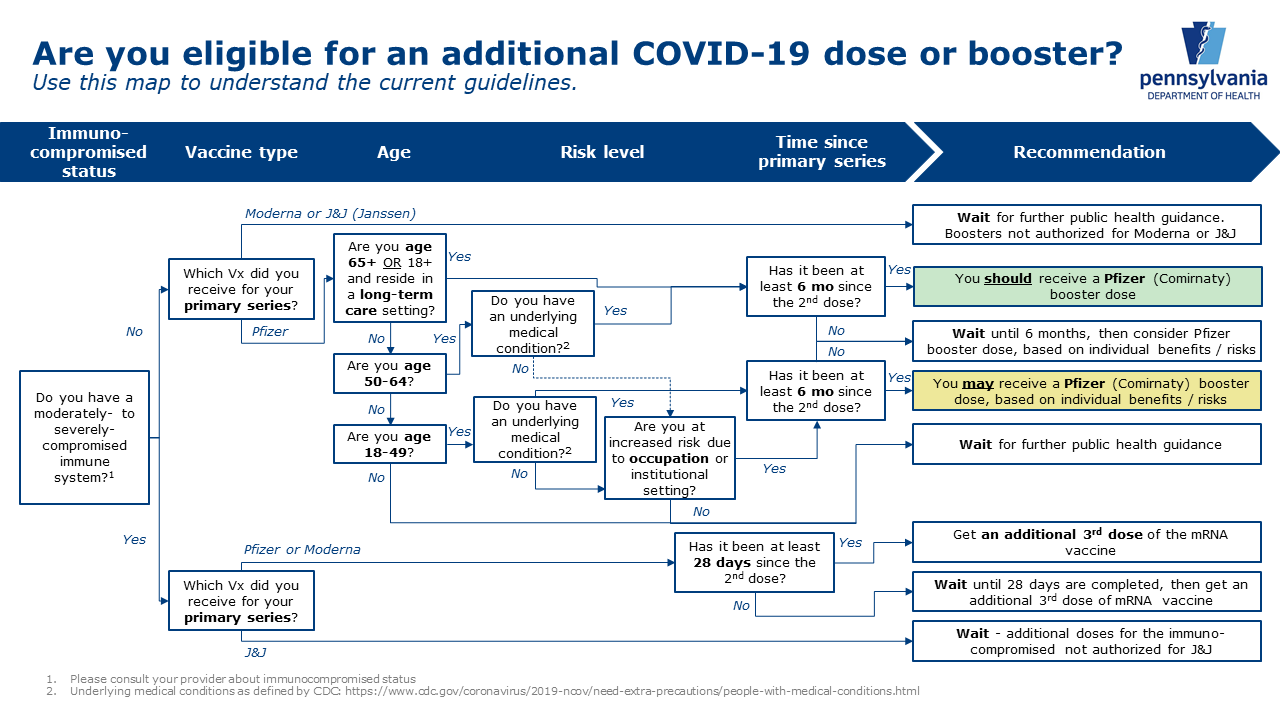
Plans for Vaccine Booster Shots from Pfizer and Moderna Being Finalized Now by FDA, CDC – AZ Dept. of Health Services Director's Blog

New COVID vaccine: FDA signs off on updated 2023 COVID booster vaccines that target XBB.1.5 Omicron subvariant, EG.5 - ABC7 New York

FDA Panel Says Pfizer COVID Booster OK For Older People And Those At High Risk : Coronavirus Updates : NPR

FDA authorizes another booster dose of the Pfizer or Moderna COVID-19 vaccine for people age 50 and up - The Boston Globe

FDA authorizes new guidance on Pfizer vaccines and boosters for children and adults : Oregon Health News Blog
FDA advisers reject Biden's plan to offer Pfizer boosters for all, embraces 3rd shots for older, at-risk Americans only - ABC News

Tense decision-making as CDC joins FDA in recommending Pfizer booster shot for 65 & up, people at high risk and those with occupational exposure to COVID-19